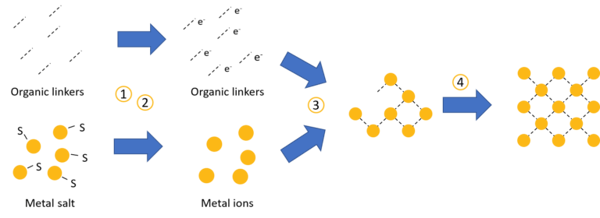
The current developments around the application of metal-organic frameworks (MOFs) for pharmaceutical and biomedical applications are forcing us to have a closer look at how harmful they are. In this post, we have a look at the toxicity of MOFs, the current status quo of research, and what’s coming.
Most metal-organic frameworks (MOFs) feature unique scaffolding structures with large pores. We usually compare MOFs with the porous structure of sponges — but in nanometer scale. We can use these pores for encapsulating other molecules, — for example active pharmaceutical ingredients or enzymes. Furthermore, the ligands – the linking molecules between the metal ions – offer open binding sites. This means, you can physico-chemically attach other molecules to the scaffolding structure of the MOFs – offering even more options to use MOFs. Various applications have been found for the pharmaceutical and cosmetics industry.
Pharmaceutical and biomedical applications of MOFs
We have seen various medical applications with MOFs being tested, while there is still no commercial product yet. This is no surprise considering the intensive clinical testing procedures required and the short existence of MOFs since their discovery in the late 1990’s.
Drug delivery
The API (active pharmaceutical ingredients) is either encapsulated in the framework, attached to the linkers or acting as the linker itself. Furthermore, the controlled release of the API can be tuned to specific durations or based on external stimuli.
MOFs as gasotransmitters
The adsorption/desorption capabilities of MOFs can be utilised as gasotransmitter, as e.g. CO is an important cell signaling mediator.
MOFs for NOx storage
The high adsorption capabilities of MOFs offer NOx storage and release for various functions in the human body.
Enzyme encapsulation
MOFs stabilise enzymes by encapsulating entire enzymes, which on their own are highly efficient and selective catalysts but unstable and hard to recycle at the same time.
Biomedical imaging
As most metal-organic frameworks decompose and can be made using bio-compatible building units, they offer great advantages over inorganic nanomaterials used for biomedical screening and imaging.
Sensing and detection
While luminescence in MOFs has been studied in various applications, research in the biomedical/pharmaceutical field is rather limited but offers great potential.
What is toxicity?
Toxicity defines the dosage of a specific substance needed to damage an organism. To determine the lethal dose, the chemical substance in question is tested on a living population in various doses. The usual measurement LD50 (lethal dose, also LC50/lethal concentration for 50% of population) the amount of a substance needed to kill half of the tested organisms. Furthermore, cytotoxicity defines the toxicity of substances on a cellular level.
| Description | LD50, mg/kg-1 |
| Extremely toxic | ≤1 |
| Highly toxic | 1-50 |
| Moderately toxic | 50-500 |
| Slightly toxic | 500-5’000 |
| Practically non-toxic | 5’000-15’000 |
| Relatively harmless | >15’000 |
Not the chemical substance itself defines (cyto-)toxicity, but rather the organism or cell that is affected by the substance. A human being might react differently to a specific substance than a laboratory animal would do. The same applies for different types of cells – one type might not react to a specific substance, while others are killed almost immediately. Therefore, results of animal tests can’t be directly transferred to the human body. That also means that LD50 measurements in various tests (including animal tests) have to be verified for humans before any drug or drug carrier is made accessible to the public.
Moreover, chemicals can be toxic immediately in minor, one-time exposure (acute toxins) or — on the opposite — over a longer period of time (chronic toxins). Acute toxins are, for example, carbon dioxide or nitrous oxide. Both are found in the human body naturally, but at higher concentrations or at a certain pressure, they can be deadly to the organism. Compared to acute toxicity, chronic toxicity takes weeks, months, or even years to damage an organism to the point of death. Understanding chronic toxicity is therefore a work of years and mostly based on statistics and epidemiology.
Testing toxicity
The smaller a particle is, the easier it will go through natural barriers such as skin, pulmonary mucosa, or even cell walls. Metal-organic frameworks are small, easily below 100 nm. Furthermore, the smaller the size and the larger the surface area, the more reactive are such particles. The surface area and the composition strongly determine interaction with the environment and dispersion. Therefore, extra care has to be taken when testing toxicity of MOFs. To do so, several test settings are used.
In-vitro
The interaction of the MOFs with cells is examined outside of the organism — in a test tube for example. In this setting, external factors can be well controlled, and the interaction is reduced to the cells at hand.
In-vivo
In contrast to in-vitro studies, in-vivo studies take place within a living organism, e.g. laboratory animals or humans.
In-silico
In-silico labels computational toxicity predictions based on various sources of information and aim to complement in-vitro and in-vivo studies. These are used in various models to predict how toxic particular chemicals may be.
MOFs and toxicity
At the current state, vast research has been done on the interaction of MOFs with their environment outside of the human body. We know, how specific MOFs such as HKUST-1, UiO-66 or MOF-841 react to their environment. Degradation in water, reaction with molecules in the air in e.g. cleanrooms or their stability in alkaline or acid solutions to name a few. However, cytotoxicity of metal-organic frameworks is another topic.
We see three points, that need to be evaluated when measuring the cytotoxicity of MOFs: the building units (metals and organic linkers), the reactivity towards various cells and the solutions used during synthesis.
Building units
Metal-organic frameworks are build from ‘building blocks, metal ions and organic linkers. As they tend to be biodegradable, we need to look at the toxicity of the base materials on their own.
Metals
Most widely used metal ions in MOFs are iron (Fe), aluminium (Al), zirconium (Zr), copper (Cu), magnesium (Mg), cobalt (Co) and nickel (Ni). Most of these metals show low toxicity. Even heavy metals are not as toxic as many think (if you’re interested, read this ).
Linkers
Many MOFs are linked with fumaric acid or E297, which is a food stabiliser and has a LD50 of ca. 10 g/kg. Another widely used linker is terephthalic acid, which is used to produce PET bottles and has a LD50 of more than 1 g/kg.
In in-vivo tests, metals and linkers didn’t show harmful accumulation in the body and were excreted within days.
So, most (exogenous) metals are not toxic, most (exogenous) linkers are not toxic. And you can opt for edible building blocks for your MOFs(materials, that are within the human body). Ergo, there are enough options to make non-toxic MOFs for your application.
Reactivity and size of particle
Broad research has been undertaken to determine the toxicity of metal-organic frameworks regarding their size and surface charge. As these are nanoparticles, they are able to distribute well in the body. Some MOFs (those below a certain nano size) even penetrate protective barriers within the organisms such as the Blood-Brain-Barrier or cell walls. While this is of great interest for medical treatment and other pharmaceutical applications, it needs to be considered to estimate possible side effects.
| MOF | Toxicity |
| MIL-53, MIL-88, MIL-89, MIL-100, MIL-101 | low toxicity |
| UiO-66, UiO-67 | low toxicity |
| Zr-fum | low toxicity |
| MOF-74 (Mg, Co, Ni) | low to no toxicity |
| MOF-74 (Cu, Mn, Zn) | high toxicity |
| ZIF-7 | low toxicity |
| ZIF-8 | low to high toxicity |
| MOF-5 | high toxicity |
| NOTT-100 | high toxicity |
| HKUST-1 | highly toxic |
From various studies, we have put together a shortlist of MOFs and their toxicity. Again, toxicity is defined by the affected organism. Therefore, some MOFs have low to high toxicity. Furthermore, it is widely accepted, that the toxicity of MOFs is mainly due to the degradation and the thereof released materials. If these are very active, the MOF is considered to be toxic. However, this toxicity can change with the functionalisation of the metal-organic framework etc.
As there are various ways to assess toxicity and currently only in-vitro and limited in-vivo assays on animals have been conducted, the published toxicity levels don’t have a universal claim and should be assessed case-by-case.
Solvents used during synthesis
 Last but not least, we need to look at how MOFs are prepared. Up to date, many synthesis methods rely on rather harmful solutions such as dimethylformamide (DMF) to dissolve metals and/or linkers. While they can be washed out of the MOF material afterwards, it might pose a threat to the final product. Depending on the solvent, even small amounts are dangerous to the organisms. Besides possible side effects from the final MOF-product, the environmental hazard of using such solvents in bulk needs to be considered by manufacturers. In the end, it’s not only about the risk for human lives, but also about the damage we might pose to our environment.
Last but not least, we need to look at how MOFs are prepared. Up to date, many synthesis methods rely on rather harmful solutions such as dimethylformamide (DMF) to dissolve metals and/or linkers. While they can be washed out of the MOF material afterwards, it might pose a threat to the final product. Depending on the solvent, even small amounts are dangerous to the organisms. Besides possible side effects from the final MOF-product, the environmental hazard of using such solvents in bulk needs to be considered by manufacturers. In the end, it’s not only about the risk for human lives, but also about the damage we might pose to our environment.
The safest solvent for high risk applications would be water, which unfortunately doesn’t work for all MOFs yet. We are currently increasing our efforts in finding more ways to synthesize MOFs in aquaeous solutions, but there’s still work to do.
In many synthesis procedures, the solvent must be washed out of the final MOF material. Again, washing the metal-organic frameworks with (deionised) water will do the trick for a broad range of them. But some need other substances to get the solvent out of the framework. Basing the synthesis on aquaeous solvents would also decrease the need for possibly harmful substances in the washing procedures and further improve the safety of the materials.
Wanted toxicity
Talking about cytotoxicity, we should mention that cancer treatment relies on the fact that the anti-cancer drug kills cancerous cells slightly better than healthy cells around them. The drug is administered with a direct IV infusion. This results in a high dose with immediate release and a wide distribution in the body. MOFs have shown great potential in targeting specific cells and even targeting specific organelles within cells such as mitochondria. Our scientific advisor Ross Forgan, professor of supramolecular and materials chemistry at University of Glasgow, has put it nicely:
“If you want to deliver specific cytotoxic cargo and it needs to get to the right part of the cell, it needs to be taken in and not immediately secreted. Targeting specific organelles I think is something that is now feasible and will be interesting.” Ross Forgan
Multiple MOFs such as NCP-1 or functionalised MOF-801 have been synthesised, so that they are able to deliver the API directly into the cancerous cells, while healthy cells are not affected. Or in other words: healthy cells won’t be killed. This is probably the future of drug delivery in cancer treatment or comparable applications.
Wrap-up
Properties of MOF can be tuned and so can their toxicity. They already look very promising for pharmaceutical applications judging from what research has been done even in in-vivo tests. Currently, we are only missing human studies to further advance applications such as drug delivery and biomedical imaging using MOFs.
For this article we used the following publications:
Validating Metal-Organic Framework Nanoparticles for Their Nanosafety in Diverse Biomedical Applications.
MOFs in Pharmaceutical Technology
A highly porous medical metal–organic framework constructed from bioactive curcumin
Toxicity of nanoscale metal organic frameworks: a perspective
Synthesis, Culture Medium Stability, and In Vitro and In Vivo Zebrafish Embryo Toxicity of Metal-Organic Framework Nanoparticles
Porous metal–organic-framework nanoscale carriers as a potential platform for drug delivery and imaging
In Vitro Toxicity Study of a Porous Iron(III) Metal‒Organic Framework
Application of the Metal–Organic Framework [Eu(BTC)] as a Luminescent Marker for Gunshot Residues: A Synthesis, Characterization, and Toxicity Study
Biomedical Applications of Metal Organic Frameworks
Metal–organic frameworks as potential drug carriers
Metal-Organic Frameworks in Biomedicine
Chapter 3 – Toxicity Tests: In Vitro and In Vivo
Cytotoxicity of nanoscaled metal-organic frameworks
In depth analysis of the in vivo toxicity of nanoparticles of porous iron(iii) metal–organic frameworks
Metal–Organic Frameworks from Edible Natural Products
Nanoparticles of Metal‐Organic Frameworks: On the Road to In Vivo Efficacy in Biomedicine
Surface-Functionalization of Zr-Fumarate MOF for Selective Cytotoxicity and Immune System Compatibility in Nanoscale Drug Delivery