Metal-Organic Frameworks (MOFs) are highly porous materials with unique properties such as the largest surface area known (up to 7000 m2/g). MOFs are highly ordered materials consisting of a metal center linked to an organic framework. These materials offer limitless construction possibilities due to the ability to choose both the metal center and the organic molecules forming the framework. Owing to this feature, MOFs are used for several applications such as gas capture and storage, energy storage, catalysis, sensors, and biomedical. In the biomedical field, MOFs are used in different ways, and we are going to exploret them in this blog.
Since the first work using MOFs in biomedicine in 2006, these materials' utilization evolved and found new methods for improving its capabilities in biological systems. The implementation of MOFs can be found for different purposes:
- Adsorption, delivery, and controlled release of therapeutic molecules
- Diagnostic imaging and disease treatment (theranostics)
Please take a look at our previous blog to find out more about these applications.
Why MOFs for drug delivery?
The scaled-down of MOFs to the nanoscale allowed its implementation as nanocarriers to deliver therapeutic agents for imaging, chemotherapy, photothermal therapy, and photodynamic therapy. Due to their high porosity, MOFs can carry molecules through different mechanisms such as adsorption (on their surface or open channels), trapping molecules inside the framework, and incorporating active molecules as part of the framework's structure.
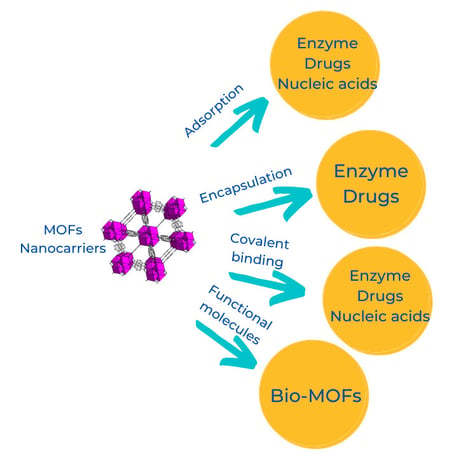
Nanocarriers functionalization strategies
Surface adsorption is the simplest and most common method used to load MOFs nanocarriers. A typical procedure consists of adding the MOF to a solution containing the active material. After an incubation time, the MOF is recovered by a simple separation from the solution. In this case, hydrogen bonds, Van der Waals, and π- π interactions are the dominant forces keeping the molecules adsorbed in the nanocarrier's surface. This method has been used to immobilize enzymes and nucleic acids in ZIFs and Ni-MOFs. MOFs allowed higher transfection efficiencies of ssDNA to mouse and human immune cells, surpassing other commercial non-viral vectors' performance.
Pore encapsulation is used due to MOFs' high porosity; this method prevents loaded molecules from leaching fast and provides a protective environment from harsh external conditions. Pore encapsulation is mainly achieved during the synthetic process; however, post-synthetic modification has also been used. This method allows the immobilization of larger molecules than the MOF pore size; it has been used to encapsulate anticancer drugs such as camptothecin and enzymes.
Another exciting feature about MOFs is their chemical tuning ability; their surface chemistry can contain functional groups such as amino, carboxyl, and hydroxyl groups. These groups can be utilized to form covalent bonds. Covalent binding aims to reduce slow leaching problems observed when adsorption and pore encapsulation methods are used. Moreover, metal nodes represent another type of reactive sites to bind functional molecules covalently. This method allows incorporating molecules like enzymes, nucleic acids, and oligonucleotides, increasing colloidal stability and enhanced cellular uptake.
What are bioMOFs?
A novel approach to increase the biocompatibility and biological activity is incorporating biomolecules as building blocks of MOFs. The MOFs containing such biomolecules as organic ligands are known as bioMOFs. Several bioMOFs have been created using molecules like amino acids, peptides, nucleobases, and saccharides. This approach will increase the biological applications; however, further research is needed to improve the stability and performance of bioMOFs.
MOFs are a promising platform for the development of drug delivery systems. Compared to other porous materials, MOFs offer several advantages:
- High surface area and porosity for high loading of therapeutic agents.
- Facile modification of physical (e.g., pore size and shape) and chemical properties of MOFs through inorganic clusters and/or organic ligands.
- Diffusion of substrates to interact with the incorporated molecules via the MOF's open windows and pores.
- Moderate strength of coordination bonds, making MOFs biodegradable.
- Well-defined structures that are beneficial for host-guest interaction.
If you are looking to explore MOFs' benefits, don't hesitate to contact us!