Carbon dioxide capture and storage is of great importance for environmental and industrial reasons. Current technologies for carbon capture based on liquid adsorbents are energy-intensive. Therefore, the latest advances in this area focus on implementing porous materials such as zeolites and Metal-Organic Frameworks (MOFs). MOFs are superior porous materials that are a promising alternative for the capture and storage of CO2 due to their high adsorption capacities and tunable selectivity for carbon dioxide. The versatility offered by MOFs for a tailored design opens the gate to new technologies that will reduce energy consumption during the adsorption-desorption process.
Current technology for CO2 adsorption-desorption
-3.png?width=154&name=Untitled%20design%20(5)-3.png)
Traditional gas liberation technologies for porous adsorbents are driven by either temperature or pressure. These technologies require large quantities of energy to achieve sufficient changes in Pressure Swing Adsorption (PSA) and Vacuum Swing Adsorption (VSA) processes. Other technologies such as Temperature Swing Adsorption (TSA) need heat sources for the operation, which are also energy-intensive. Although waste heat can be used, porous materials are often thermal insulators and this dramatically reduces the overall efficiency of the process. For these reasons, a novel solution is required where the porous material can be regenerated with reduced energy consumption.
Novel gas release methods
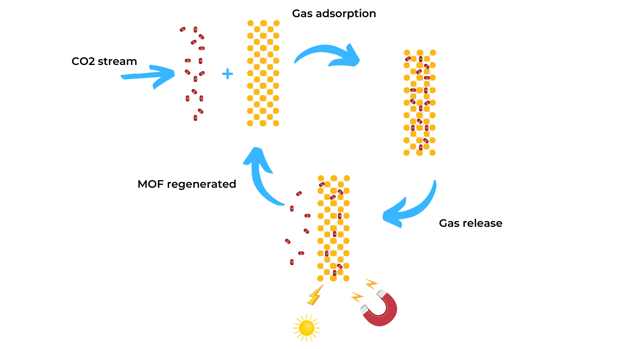
MOFs are a versatile platform due to the tunability of their chemistry where they allow for the incorporation of molecules with novel properties that can respond to external stimuli such as light or a magnetic field. This stimuli can trigger the release of gas adsorbed in the porous materials. Taking advantage of the responsiveness of MOFs, alternative methods to VSA, PSA, and TSA processes have been developed which are known as Light Induction Swing Adsorption (LISA) and Magnetic Induction Swing Adsorption (MISA).
- In the case of LISA, light-responsive motifs are introduced in the MOF structure which trigger the release of gas at low temperature and pressure (278 K and 1 bar). Light-sensitive MOF adsorbents can use either sunlight or UV light as an energy source. In particular, sunlight is an easily accessible, renewable and cheap energy source that can used in carbon capture applications.
- For the MISA process, magnetic nanoparticles are introduced inside the framework, activated by applying a magnetic field that triggers the CO2 release. This process is highly efficient and overcomes the issue of poor thermal conductivity within the adsorbent.
Achieving high release efficiencies

The implementation of light-triggered gas release from MOFs has been tested using carbon dioxide adsorption measurements, achieving up to 90.5% of CO2 desorption at 1 bar; this process can be made in only 14 min under visible light irradiation.
MISA technology offers the possibility to trigger gas release remotely, which is more advantageous for large-scale applications. The desorption capacity of CO2 in magnetic MOF adsorbents can reach up to 89.4% at 1 bar, comparable to the efficiencies reported for LISA technology. More recently, researchers explored a combined approach by adding a UV light source for the desorption process of magnetic MOFs. The so-called MaLISA method increased the desorption capacity, achieving a 96.8% release of the total CO2.
The energy required for the regeneration step of the adsorbent is a critical aspect of evaluating the viability of a gas capture technology. Mature technologies like liquid amine adsorbents using a TSA process require about 3 MJ per kg of CO2 processed, while MOF-based systems can reduce energy costs by up to 80%. The implementation of new technologies can allow for the use of renewable energy sources, reducing our dependency on fossil-fuels.
If you want to know more about MOFs, please visit our previous blogs about carbon capture applications.




.jpg)
