Gas storage technology is critical for implementing alternative fuels such as hydrogen and methane to combat pollution due to greenhouse gas emissions. A suitable technology will allow capturing and storage of CO2 as well. An important feature that needs to be improved in order to implement MOF-based systems is the volumetric working capacity under real operating conditions. Recent advances show promise towards the practical use of MOFs for gas storage at a large scale.
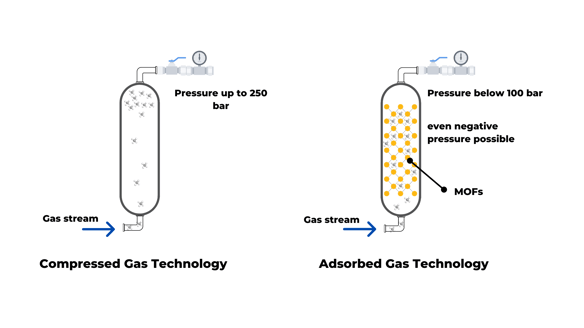
Current gas storage technologies are based on compression, using high pressures (250 bar). In the case of hydrogen, the available technology also requires low temperature due to its unique physical-chemical properties, making the storage process more energy-consuming. Implementing more environmentally friendly fuels is not only needed for the transportation sector; other industrial processes can take advantage of alternative fuels. In other words, more efficient gas storage technologies are necessary to allow applications in the near future.
What is adsorbed gas technology?
The adsorbed gas technology emerged as an alternative to conventional techniques to decrease the pressure and energy needed for storage. This method relies on porous materials to retain the gas molecules. Metal-Organic Frameworks (MOFs) have shown remarkable storage capacity among the absorbent materials and have been extensively studied in the last decade.
 MOFs are materials with high porosity and ultrahigh surface area. They have gained attention for gas storage since they offer advantages as tunable pore size and shape and chemical tailoring to increase molecule interactions. MOFs like HKUST-1 possess pore sizes below 2 nm and exhibit volumetric methane uptake as high as 200 cm3 (STP)/cm3 at 65 bar.
MOFs are materials with high porosity and ultrahigh surface area. They have gained attention for gas storage since they offer advantages as tunable pore size and shape and chemical tailoring to increase molecule interactions. MOFs like HKUST-1 possess pore sizes below 2 nm and exhibit volumetric methane uptake as high as 200 cm3 (STP)/cm3 at 65 bar.
Despite the great potential of MOFs, there still some challenges to overcome to achieve practical applications. Their highly porous nature makes them ideal for increasing the storage capacity; however, these materials' microstructures lack physical stability for long-term operation. Therefore, there is a need to increase MOF-based systems' strength to avoid their microstructure collapsing at real application conditions.
Tuning MOFs' macrostructure
One of MOFs' most exciting advantages is making a conscious design regarding pore size and shape. This goal can be achieved by changing the framework's components (using different metal nodes and organic ligands). Often MOFs are obtained as powders with a small grain size (less than 0.5 mm). When these powders are used in tanks, they need to be compacted to decrease the void space between grains, which leads to a volumetric capacity loss. In standard practices, compaction includes pressure densification or chemical binder addition to improve the pellets' mechanical properties. However, these compaction techniques can also lead to the microstructures' collapse, again lowering the volumetric working capacity.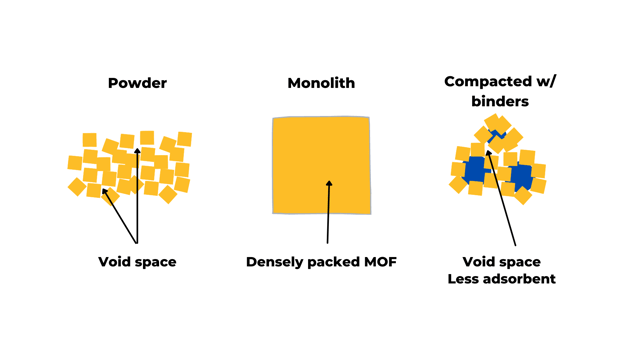
But what about the macrostructure? For this matter, the manipulation of the synthesis protocol can offer control at a larger size. In a recent report, a group of researchers explored the effect of the solvent's temperature during fabrication on MOFs' macrostructure. According to this report, when the solvent is removed at a lower temperature, it creates larger structures in the range of centimeters; the resulting structures are called monoliths. They were able to fabricate monoliths of the MOF UiO-66 when the temperature was decreased from 200 °C to 30 °C. UiO- 66 (Zr6O4(OH)4(1,4-benzenedicarboxylate)6 was chosen due to its high thermochemical and mechanical stability, desirable features for industrial applications.
Volumetric working capacity of 172 cm3 (STP)/cm3 (MOFs) vs. 135 cm3 (STP)/cm3 other porous materials
The modifications made during fabrication allowed the formation of a mixed micro/mesoporous MOF. As a result, significant improvement in the gravimetric uptake capacity was observed of 0.252 g/g (298 K, 100 bar) for CH4 and 0.670 g/g (298 K, 40 bar) for CO2. Another significant achievement was gas uptake's ability even at the maximum pressure, which is not the case for powders. The uniform uptake capacity at different pressures is translated as a continuous delivery during operation, a critical feature necessary for vehicles' application.
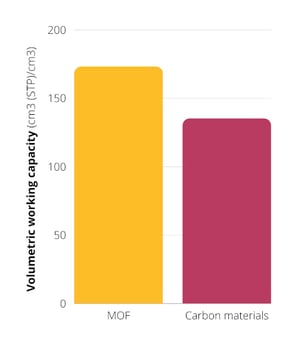
For practical applications, the volumetric working capacity is used as a reference value, representing a more accurate value of the system's actual delivery capacity. This term is the difference between the uptake capacity at maximum and the release pressure (usually 65 and 5 bar recommended by the US Department of Energy). Although other materials like zeolites and nanocarbon materials can show similar values in volumetric uptake; The UiO-66 MOF showed a volumetric working capacity value of 172 cm3 (STP)/cm3 at 65 bar, while others like nanocarbon materials reach 135 cm3 (STP)/cm3 without compaction.
In summary, MOF-based systems offer valuable advantages for adsorbed gas storage technology such as:
- High microporosity
- Weak interactions with methane
- Continuous uptake capacity in all the pressure operation range
- Enhanced volumetric working capacity
- A balance between surface area, porosity, and density
These recent advances set the MOF-based systems as the most promising technology for real applications in the transportation and industrial sectors. More information about MOFs' implementation as gas storage systems can be found in our case study for methane storage from natural gas.
Are you looking to explore the world of MOFs?
Don't hesitate to contact us! We can help you choose the right MOF for your application.



