Post-combustion carbon capture technologies are critical for reducing air pollution in the upcoming years. The major drawback of mature carbon capture technologies is their energy-intensive nature. For this matter, alternative technologies have emerged to increase their efficiency as much as possible. This blog compares the existing technologies to offer an overview of this area.
Post-combustion carbon capture
 In post-combustion capture, carbon dioxide is captured from flue gas streams produced during fossil fuel combustion. Besides CO2, the flue gas stream contains other pollutants, such as nitrous oxides and sulfur oxides, which need to be removed to allow the reutilization of the stored CO2.
In post-combustion capture, carbon dioxide is captured from flue gas streams produced during fossil fuel combustion. Besides CO2, the flue gas stream contains other pollutants, such as nitrous oxides and sulfur oxides, which need to be removed to allow the reutilization of the stored CO2.
The most popular technologies for post-combustion carbon capture are amine scrubbing, calcium looping, and adsorption with porous materials such as zeolites, carbon materials, and Metal-Organic Frameworks (MOFs).
Amine-based technologies
Amine scrubbing uses liquid amine-based solvents to capture CO2. Amines are organic compounds with high CO2 solubilities and fast reaction rates. Amine solvents like monoethanolamine (MEA) and diethanolamine (DEA) are put into contact with flue gases inside a reactor where CO2 is transferred to the liquid. After absorption, CO2 is released from the amine solvent in a steam reactor with a higher temperature between 120 – 150 °C. At higher temperatures, the CO2 solubility decreases, allowing solvent regeneration for a new capture cycle.
The main cost factor in CO2 capture with amines technology is the energy required for the stripping step. The energy requirement in current facilities is around 2.6 GJ per metric tonne of CO2 captured . Besides the high energy requirements and the high cost of amine solvents, another drawback of this method is the presence of corrosive compounds in flue gas streams that degrade amines resulting in solvent loss, equipment corrosion, and generation of volatile, toxic degradation compounds. The factors mentioned above are holding the extensive deployment of this technology.
Calcium looping technology
Calcium looping is an alternative technology that uses materials with a lower cost (such as limestones and dolomites) that are more environmentally friendly than amines. The Ca-looping process uses a CaO-based material that can undergo a reversible reaction with CO2. The CaO material reacts with CO2 to form calcium carbonate (known as carbonation, and the reversed reaction is called calcination); this step is temperature-dependent (650-700 °C), and at a higher temperature, more CaCO3 can be formed.
After capture, CO2 is released in the calcination step by increasing the temperature in the reactor to 900-950 °C. The heat necessary for this process is considerably high; therefore, the energy consumption is also high, which is the major drawback of Ca-looping technology.
Adsorption using porous materials
Owing to the high energy requirements of the most mature methods for post-combustion carbon capture (such as amine scrubbing and Ca-looping), the implementation of porous materials arose to achieve a more energy-efficient carbon capture method. Amongst porous materials, zeolites and carbon materials are the most used.
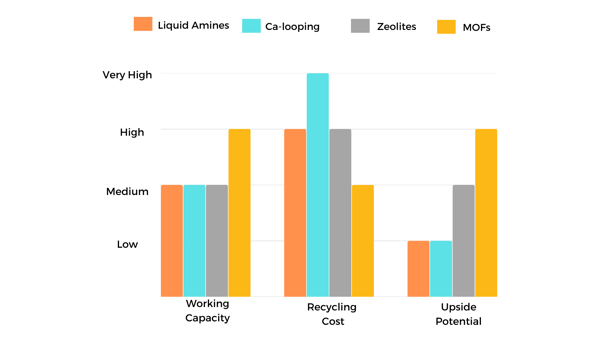
Natural or synthetic Zeolites possess good thermal stability with moderate CO2 adsorption capacities. An important disadvantage of these materials comes from their hydrophilic nature; zeolites can lose up to 60-78.5% uptake capacity in the presence of water when used for CO2 capture in flue gas streams due to the competitive adsorption of CO2-H2O.
Carbon materials such as activated carbon are cheap and widely available. However, they have lower CO2 adsorption capacities than zeolites meaning they would need a larger volume to achieve similar adsorption capacity.
 A relatively new kind of materials are Metal-Organic Frameworks; compared to other porous materials, they possess higher surface areas due to their super-high porosity. MOFs consist of metal centers joined by organic linkers, which can be chosen from a tremendous number of options allowing almost infinite combinations. This feature enables the possibility to manipulate the pore size and shape and the surface chemistry inside the pores. Due to its tunable chemistry, hydrophobic MOFs can be fabricated to reduce the effect of high humidity levels while maintaining their CO2 adsorption capacity unaffected.
A relatively new kind of materials are Metal-Organic Frameworks; compared to other porous materials, they possess higher surface areas due to their super-high porosity. MOFs consist of metal centers joined by organic linkers, which can be chosen from a tremendous number of options allowing almost infinite combinations. This feature enables the possibility to manipulate the pore size and shape and the surface chemistry inside the pores. Due to its tunable chemistry, hydrophobic MOFs can be fabricated to reduce the effect of high humidity levels while maintaining their CO2 adsorption capacity unaffected.
Advanced physi-sorbents such as MOFs that selectively capture CO2 from flue gas streams offer the most significant upside potential for a revolution in carbon capture technology since they would require much less energy for regeneration and exhibit high working capacity, if appropriately designed, will be unaffected by moisture.
Material regeneration cost
The material regeneration cost is crucial for future industrial implementation of carbon capture technologies. The energy required is dependent on the temperature used for CO2 release. As mentioned before, in amine scrubbing technology, the stripping step temperatures vary between 120- 150 °C. Although the temperatures are not so high, the high volume used by amine scrubbers increases the energy needed for the regeneration step. Calcium looping is the most energy-intensive process, which requires temperatures above 800 °C for the regeneration step.
Zeolite-based technology requires mild temperatures around 150 °C. However, zeolites suffer from an efficiency drop in high humidity conditions after a few cycles. Among porous materials, activated carbon and MOFs are the materials that require lower energy inputs for regeneration since they need temperatures below 90°C. However, activated carbon has considerably lower adsorption capacities than the other materials. MOFs have shown good performance even in high humidity conditions.
Waste heat utilization is a cost-effective option to power CO2 capture. The low energy requirement of MOF materials opens new opportunities to take advantage of waste heat (which typically has a temperature around 120 °C). If waste heat is used for carbon capture, the operation cost can be reduced drastically. The cost of generating steam from waste heat is about 8-fold lower than the cost of steam from an existing natural gas-fired boiler which has been studied in industries like cement, silicon, iron & steel, and pulp & paper.
Are you interested in MOFs? Please visit our previous blogs and success stories to keep exploring the benefits of MOF materials for carbon capture.




.jpg)
