The theme of World Environment Day 2022 is 'Only One Earth'. The campaign highlights the need to change to a cleaner and greener lifestyle. On the path to a sustainable life, the world needs to decrease the emission of pollutants to the atmosphere derived from human activities. Since carbon dioxide is the most abundant greenhouse gas in the atmosphere, Carbon Capture and Storage (CCS) has become critical to achieving this challenging task. This blog covers the key aspects of achieving successful CCS using porous adsorbents, an emerging method showing promising results in the field.
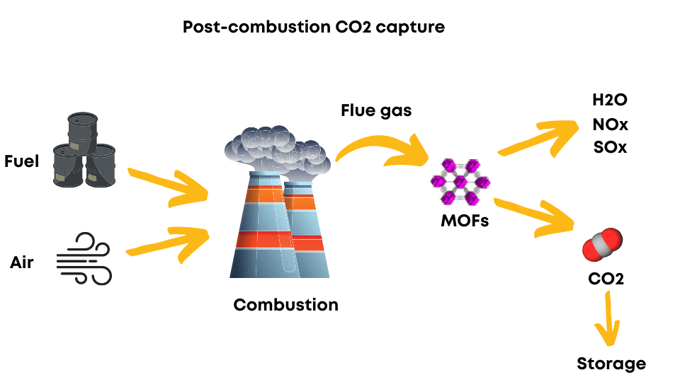
Post-combustion carbon capture
Carbon Capture can be made in Oxy-fuel combustion, pre-combustion, and post-combustion. From these methods, post-combustion is favored to sequester CO2 from flue gas. Flue gas streams after the combustion of fossil fuels contain around 15% CO2 with a partial pressure range of 0.13-0.16 bar at 40-60 °C. Due to the high moisture, low CO2 concentration, and the presence of corrosive gases such as NOx/SOx in the flue gas stream, big challenges arise for the stability and efficiency of porous solid adsorbents.
Among the different methods for carbon capture, the absorption with liquid amines such as monoethanolamine (MEA) dominates the scene. In this so-called chemical adsorption technique, CO2 scrubbers based on liquid amines use the reversible reactions between carbon dioxide and amines. However, the major drawback of large-scale amine scrubbers is their low energy efficiency. Therefore, alternative methods are required to achieve widespread carbon capture.
CO2 capture by the solid adsorption method involves trapping it on the surface of a porous adsorbent such as activated carbon, zeolite, or Metal-Organic Framework (MOF). Activated carbons and their derivatives are versatile materials with high thermal stability, availability, and low cost. Zeolites are natural molecular sieves possessing a high affinity toward acidic gases. These two materials are the most used for CO2 capture since they have been employed on a large scale. Unlike activated carbons and zeolites, MOFs are new hybrid materials, offering easy tunability of the surface chemistry and pore size with exceptionally high surface areas. MOFs have shown great potential for CO2 capture with outstanding selectivity.
Enhanced material stability
The presence of water in flue gas streams along with corrosive nitrogen and sulfur compounds requires the high stability of the porous solid adsorbents. While achieving high thermal and chemical stability in porous adsorbents like activated carbons and zeolites is relatively easy, this can represent a significant challenge in the case of MOFs.
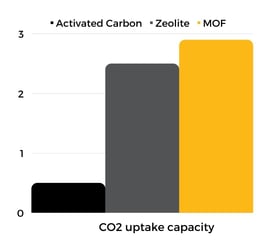 Recent advances in MOF materials have shown outstanding performance with a superior CO2 uptake capacity (at low-pressure conditions) and lower regeneration energy requirements for carbon capture from flue gas. For instance, a novel MOF achieved an uptake capacity of 65.7 cm3/g at 0.1 bar pressure and 101.2 cm3/g at 1 bar (298K). Stability tests of this MOF in the presence of high humidity and corrosive molecules showed that its CO2 uptake capacity was retained up to 99% (74 % RH humidity conditions, and storage in a 0.05% SO2, 0.005% NO2, 10% CO2, and 89.945% N2 mixture at 298 K), evidencing an increase in chemical stability of these hybrid materials. MOFs showed promising results outperforming typical porous adsorbents under flue gas stream conditions, making them ideal for CO2 capture (see figure below). For more examples of MOF’s applications for carbon capture, please visit our success stories.
Recent advances in MOF materials have shown outstanding performance with a superior CO2 uptake capacity (at low-pressure conditions) and lower regeneration energy requirements for carbon capture from flue gas. For instance, a novel MOF achieved an uptake capacity of 65.7 cm3/g at 0.1 bar pressure and 101.2 cm3/g at 1 bar (298K). Stability tests of this MOF in the presence of high humidity and corrosive molecules showed that its CO2 uptake capacity was retained up to 99% (74 % RH humidity conditions, and storage in a 0.05% SO2, 0.005% NO2, 10% CO2, and 89.945% N2 mixture at 298 K), evidencing an increase in chemical stability of these hybrid materials. MOFs showed promising results outperforming typical porous adsorbents under flue gas stream conditions, making them ideal for CO2 capture (see figure below). For more examples of MOF’s applications for carbon capture, please visit our success stories.
Porous adsorbents for CO2 capture
The promising results for CO2 capture from flue gas with MOFs have set a new benchmark, showing the progress in the field. Besides the high uptake capacity and good selectivity shown by MOFs, the energy efficiency is critical for its implementation at the industrial scale. The heat of CO2 adsorption is related to the energy necessary to release the captured carbon dioxide. The heat of adsorption in promising CO2 adsorption MOFs is as low as 38.2 kJ/mol, which is considered efficient for the adsorption-desorption cycling process.
The low energy consumption of MOF-based systems improves the overall process efficiency compared to well-established technologies such as aqueous amines, which have a typical heat of adsorption of 105 kJ/mol, decreasing the energy consumption of regeneration by up to 2.7 times.
The performance of MOF adsorbents is clear evidence that, finally, adsorption using solid porous adsorbents matched the CO2 capture capacity of well-established technologies based on liquid adsorbents. Therefore, solid porous adsorbents will play a crucial role in future carbon capture and storage technologies.




.jpg)
