Implementing carbon capture and storage systems is critical to reducing environmental pollution. Current capture technologies are based on liquid amine absorption methods (amine scrubbing). Besides amines, calcium looping systems have been developed to increase carbon capture efficiency. Alternatively, adsorption methods are gaining interest as an excellent option to replace absorption processes. Adsorption technology uses solid, porous materials to trap CO2. It is intended to be implemented mainly in fossil fuel power plants to capture the CO2 generated after combustion. Nevertheless, adsorption technology is facing some challenges which novel materials could overcome.
Challenges in carbon capture technologies
 Amine scrubbing is a well-established technology used at the industrial scale for carbon capture due to its good CO2 efficiency and technology maturity. However, it is an energy-intensive technique that significantly reduces the overall energy efficiency of the carbon capture system. For instance, the capture step in an amine-based system for a coal-fired power plant accounts for 60-80% of the system's total cost. Moreover, the solvents used in amine scrubbers are highly corrosive, leading to a fast deterioration of the equipment.
Amine scrubbing is a well-established technology used at the industrial scale for carbon capture due to its good CO2 efficiency and technology maturity. However, it is an energy-intensive technique that significantly reduces the overall energy efficiency of the carbon capture system. For instance, the capture step in an amine-based system for a coal-fired power plant accounts for 60-80% of the system's total cost. Moreover, the solvents used in amine scrubbers are highly corrosive, leading to a fast deterioration of the equipment.
Calcium looping (or Ca-looping) is another chemical reaction method based on calcium materials derived from limestones. The sorbent materials are cheap and possess a high affinity towards sulfur compounds which is beneficial for the production of a pure CO2 gas stream. Nevertheless, Ca-looping reactions are temperature dependent and require temperatures as high as 900-950°C. Therefore, the process is energy-intensive and suffers from low cycling stability. Owing to these disadvantages, new materials are under development to increase the process efficiency.
The most novel and exciting alternative for carbon capture is adsorption technology. Adsorbents with high porosity are used to increase the capture capacity. The porous materials with promising results are zeolites and Metal-Organic Frameworks (MOFs).
Is adsorption technology a good alternative?
The adsorption-based methods are attractive for carbon capture due to the simplicity of the process and flexibility during operation. Since the adsorption is carried out in the solid state, the equipment necessary for operation does not suffer from excessive corrosion from aggressive solvents, and the operating costs are reduced due to their mild operation conditions.
Adsorption technology is favored for CO2 capture in post-combustion conditions such as low pressure, mild temperatures, and low carbon dioxide concentration, where implementing absorption techniques will result in high operating costs. Therefore, this technology can be implemented in two sectors with higher contribution to CO2 emissions: the industrial (i.e., cement, iron and steel, and refineries) and energy and heat generation sectors (i.e., coal and natural gas fire power plants).
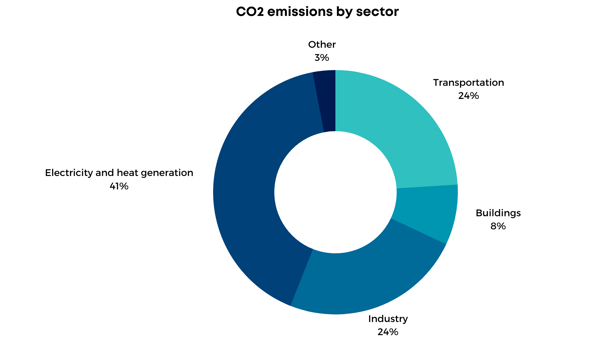 Carbon capture in coal-fired power plants
Carbon capture in coal-fired power plants
Coal is the largest contributor to carbon dioxide emissions in the world. Coal-fired power plants supply about 27% of the primary energy and generate almost 50% of global CO2 emissions. Flue gas from coal combustion contains 70-75% N2, 10-15% CO2, 8-10% H2O, 3-4% O2 and traces of SOx and NOx compounds. High-water content in this flue gas represents the major challenge during the carbon capture process due to its competitive adsorption with carbon dioxide.
Most adsorbent materials show a significant decrease in CO2 adsorption when they are in the presence of water; a capacity loss could be as high as 90%. Recent advances have been made to increase capture capacity by integrating amine molecules into adsorbents; with this modification, materials show acid-base properties like liquid amines.
Zeolites vs. MOFs
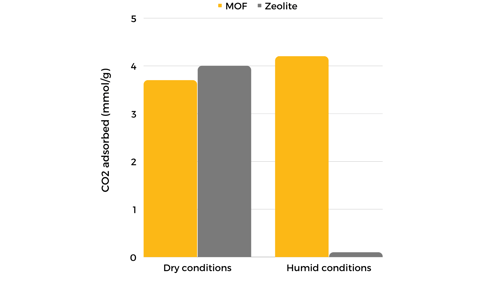
Recently, the performance of alkylamine-modified adsorbents, including zeolites (i.e., zeolite 13X), mesoporous silicas, and MOFs (i.e., mmen-Mg(dobpdc)), was assessed. Results show that zeolites and MOFs perform better under dry conditions. However, under humid conditions like those found in flue gas from coal-fired power plants, MOFs outperform zeolites, even increasing their performance slightly in the presence of water molecules.
 Stable performance of MOFs
Stable performance of MOFs
The presence of SOx, NOx, and particulate matter in flue gas streams from coal-fired power plants represents another challenge for post-combustion capture. The presence of such molecules affects not only the performance of porous materials but amine solutions too. Amines suffer from deterioration in the presence of SOx molecules leading to irreversible capture capacity loss.
Thanks to the versatility of MOF technology, MOFs can be tuned to withstand humid SO2 conditions. In comparison to aqueous amine technology, MOFs offer:
- Enhanced stability
- Superior selectivity over nitrogen
- Lower energy requirements
- Comparable working capacity
The implementation of adsorption technology can help to reduce up to 40% of the parasitic energy during carbon capture compared to typical systems such as amine scrubbing, improving the overall efficiency of the process.
Please visit our previous blogs and success stories to learn more about MOFs' amazing world.




.jpg)
