Implementing porous adsorbent materials for carbon capture has gained importance in recent years. Adsorption technology is considered a promising alternative to replace typical absorption methods based on liquid amines. However, not all porous adsorbents perform equally. Here we will discuss the pros and cons of the most used materials.
Adsorption technology for carbon capture
 The adsorption-based methods are attractive for carbon capture due to the simplicity of the process, mild operating conditions, flexibility during operation, low operating cost, and no corrosion in equipment. Adsorption technology is favored for CO2 capture in post-combustion conditions such as low pressure, mild temperatures, and low carbon dioxide concentration, where implementing absorption techniques will result in high operating costs.
The adsorption-based methods are attractive for carbon capture due to the simplicity of the process, mild operating conditions, flexibility during operation, low operating cost, and no corrosion in equipment. Adsorption technology is favored for CO2 capture in post-combustion conditions such as low pressure, mild temperatures, and low carbon dioxide concentration, where implementing absorption techniques will result in high operating costs.
When used for CO2 adsorption, solid porous materials offer high uptake capacity, easy recovery process, resistance to humid conditions, and high stability. Porous adsorbents for CO2 capture include zeolites, mesoporous silica, carbon-based materials, organic polymers, metal oxides, and hybrid materials such as Metal-Organic Frameworks (MOFs). Among these materials, zeolites and MOFs are the most exciting options for carbon capture due to their high uptake capacity.
Best features of porous absorbents
The uptake capacity is not the only significant feature of adsorption technology. Other features like adsorption kinetics (how fast they can adsorb the target molecule), selectivity, and adsorption/desorption temperature are also crucial for an efficient capture process.
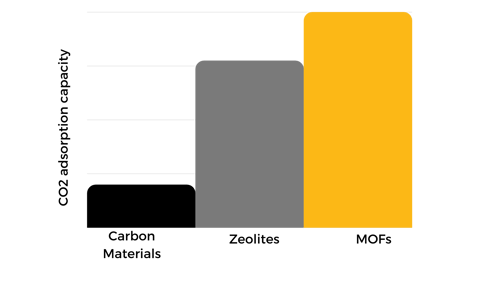
A porous adsorbent's uptake capacity and selectivity are related to its specific surface area and surface chemistry. When comparing the surface area per unit weight: the activated carbons are 400–2000 m2/g, zeolites up to 1500 m2/g, and MOFs are in the 1500–7000 m2/g range. MOFs are the porous materials with the highest surface area with an ultra-high porosity of up to 90% of free volume. MOFs' surface area can be easily tuned during the material's design through the selection of metal ions and organic ligands.
The ability to change the surface chemistry in Metal-Organic Frameworks is superior to those of carbon materials and zeolites. The manipulation of MOFs structures includes pre- and post-synthetic modifications such as phosphonate, amine, and sulfonate functionalization, multifunctional ligands, mixed ligand-functionalization, and ethylenediamine functionalization, among others. The tuning of the chemical nature of MOFs makes it possible to increase the performance in the carbon capture process. Therefore, MOFs show a superior CO2 adsorption capacity compared to other adsorbents like carbon materials and zeolites.
 Another critical property of porous adsorbents is the adsorption-desorption temperature which is directly related to energy consumption during operation. Zeolites, carbon materials, and MOFs work in the low-temperature range for the adsorption process (within the typical range of flue gas under 100 °C). However, in the desorption step (where the porous material is regenerated), zeolites usually require higher temperatures, around 300 °C, making the process more energy intensive. Conversely, carbon materials and MOFs need lower temperatures (about 100 °C) to be regenerated and ready for another adsorption cycle. The low energy requirement of MOFs for the desorption step, combined with their high uptake capacity, makes them a better option for CO2 capture.
Another critical property of porous adsorbents is the adsorption-desorption temperature which is directly related to energy consumption during operation. Zeolites, carbon materials, and MOFs work in the low-temperature range for the adsorption process (within the typical range of flue gas under 100 °C). However, in the desorption step (where the porous material is regenerated), zeolites usually require higher temperatures, around 300 °C, making the process more energy intensive. Conversely, carbon materials and MOFs need lower temperatures (about 100 °C) to be regenerated and ready for another adsorption cycle. The low energy requirement of MOFs for the desorption step, combined with their high uptake capacity, makes them a better option for CO2 capture.
Regarding material cost, MOFs are behind zeolites and carbon materials, making carbonaceous materials the cheapest option. However, the development of new methods for MOF synthesis is gaining interest. The MOF market is growing fast, with an expected compound annual growth rate of nearly 34.3% over the next five years. MOFs will soon become cheaper and a viable economic option for industrial carbon capture in industries like cement and steel.
Conclusions
Adsorption technology has become a promising alternative to absorption methods due to the advances in the development of porous adsorbent materials. Adsorption methods can be designed to work with waste heat due to the low energy requirement for adsorbent regeneration. New hybrid materials such as Metal-Organic Frameworks with outstanding uptake capacities make post-combustion carbon capture an economically viable option.
Please visit our previous blogs and success stories to learn more about MOFs' amazing world.




.jpg)
