Point-source carbon capture technologies have emerged as a critical solution for combating climate change by reducing the amount of carbon dioxide (CO2) emissions released into the atmosphere. As nations around the world strive to meet ambitious emission reduction targets, carbon capture has become a focal point in the quest for sustainable energy practices. Among the many carbon capture methods, absorption and adsorption technologies stand out as promising ways to capture CO2 emissions from various industrial sources. Here, we delve into the world of carbon capture, with a particular focus on absorption and adsorption methods, and discuss their importance in shaping a greener future.
Absorption: A Foundation in Carbon Capture
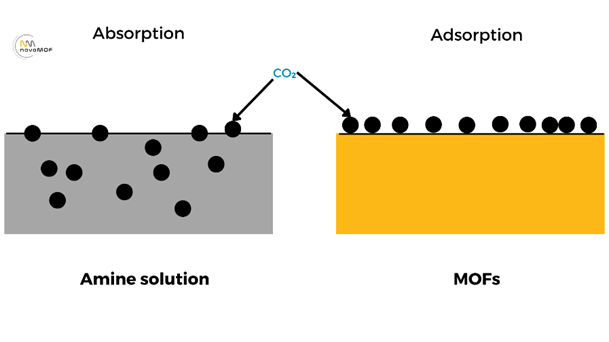
Absorption-based technologies play a central role in carbon capture, providing an effective means of capturing CO2 from flue gases emitted by power plants, industrial facilities, and other sources. The process involves passing the CO2 rich gas stream through a liquid solvent, typically an amine solution, where the CO2 is chemically absorbed. The solvent, now loaded with CO2, is then processed to separate the captured CO2 for storage or utilization.
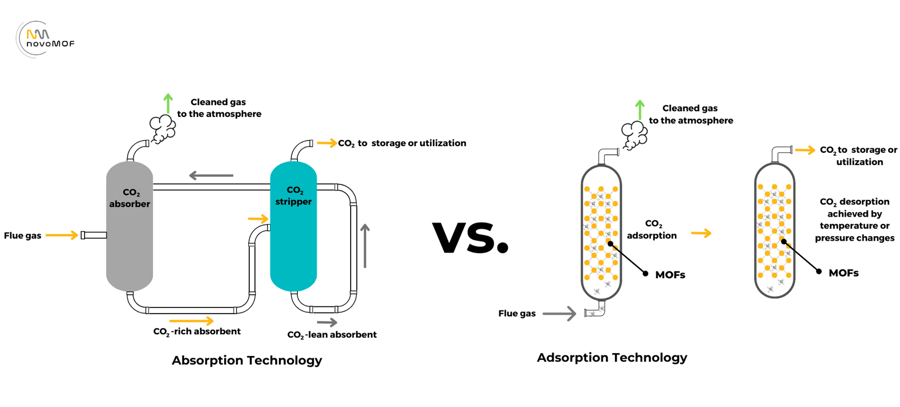
Amines, such as monoethanolamine (MEA) and diethanolamine (DEA), are commonly used as absorbents due to their high affinity for CO2. These materials undergo reversible chemical reactions with CO2, facilitating effective capture and release processes. Absorption systems typically consist of absorbers, strippers, and regeneration units where the solvent is returned to the absorber for reuse.
A significant advantage of absorption-based carbon capture is its maturity and widespread application in industrial settings. In addition, absorption systems can achieve high capture rate, making them attractive options for large-scale deployment. However, challenges such as solvent degradation, energy-intensive regeneration processes, and potential environmental impacts limit the widespread adoption of absorption technologies.
Adsorption: Harnessing Porous Materials for Carbon Capture
Adsorption processes using porous materials such as zeolites, activated carbon, and Metal-Organic Frameworks (MOFs), represent an innovative approach to carbon capture. Unlike absorption, where CO2 is dissolved in a liquid, adsorption involves the physical entrapment of CO2 molecules onto the surface of a solid adsorbent material.
The widespread adoption of adsorption processes using materials like zeolites and activated carbon faces several challenges that have prevented their use in point-source CO2 capture. Activated carbon suffers from poor selectivity, and the performance of zeolites is significantly impacted by moisture, necessitating a costly extra drying step.
Recently, MOF-based materials have emerged as a promising alternative to typical adsorption materials. Characterized by their high surface area and tunable pore structures, MOFs exhibit exceptional CO2 adsorption capacities. These crystalline materials consist of metal ions or clusters connected by organic linkers, creating nanoporous frameworks ideal for CO2 capture. Tuning the chemical structures of MOFs enables selective adsorption mechanisms, allowing them to effectively capture CO2 from gas streams, offering a promising solution for carbon capture applications.
Adsorption-based carbon capture using MOFs has several advantages. MOFs offer tunable properties, allowing for the design of adsorbents tailored to specific CO2 capture requirements. Furthermore, MOF-based adsorption processes typically operate at lower temperatures (as low as 90° C) than other materials in the regeneration step (typically in the range 120-150°C), reducing energy consumption.
The Future of Carbon Capture
Recent advances in the field of MOFs have shown promise in overcoming typical limitations of adsorbent materials, such as moisture sensitivity and poor selectivity (zeolites and carbon-based materials), through tailored structural design. By fine-tuning the composition and pore geometry of MOFs, researchers have successfully designed stable and high-performance materials. MOF-based systems can meet the 95% purity and 90% recovery targets proposed as standards for efficient carbon capture at different concentration levels without pre-drying or a desiccant pre-layer. This ability to mitigate moisture sensitivity is a significant advantage over other adsorbents such as zeolites.
MOFs exhibit robustness and durability, making them attractive candidates for carbon capture applications. As a result, the tailored nature of MOF structures not only increases their effectiveness in CO2 capture but also enhances their practicality and reliability in diverse environmental conditions, further solidifying their position as a leading contender in the field of adsorption-based carbon capture technologies.
As the world seeks more sustainable solutions to mitigate CO2 emissions, adsorption processes, particularly those employing MOFs, hold great promise for the future of carbon capture. Continued research and development efforts to improve the stability, scalability, and cost-effectiveness of MOFs drive their widespread adoption in industrial carbon capture applications. By harnessing the power of porous materials, we can pave the way to a greener, more sustainable future for generations to come.
The D and the B in carbon capture, referring to adsorption and absorption methods, respectively, play a critical role in addressing the global challenge of climate change. While absorption technologies offer proven reliability and effective, adsorption methods, particularly those utilizing MOFs, hold immense potential for revolutionizing carbon capture. By understanding the strengths and limitations of each approach and embracing innovation, we can accelerate progress toward a carbon-neutral future.
For further insight into the world of MOFs, explore our previous blogs and success stories.




.jpg)
