Climate change is one of the most important topics in our society. The effects of climate change are evident, and they require action from all of us to help reduce catastrophes such as droughts and hurricanes. In this sense, efforts from people worldwide gave birth to Earth Day in 1970, celebrated on 22nd April every year. This year's celebration focuses on a call-to-action from the private sector to reduce pollution associated with human activities in the industry. In order to reduce emissions from fossil fuels, the world is transitioning to renewable energies. However, during this transition phase, we still need to decrease emissions to the atmosphere. This blog highlights the importance of CO2 capture, which is crucial in reducing the greenhouse gas effect.
Why CO2 capture?
 Carbon dioxide plays a significant role as greenhouse gas in the atmosphere. Its generation is related to human activities such as transportation and industry operation. Combustion processes are used for energy generation in the industry sector, and flue gases are released. Carbon capture during post-combustion processes is considered a viable option to help reduce CO2 emissions.
Carbon dioxide plays a significant role as greenhouse gas in the atmosphere. Its generation is related to human activities such as transportation and industry operation. Combustion processes are used for energy generation in the industry sector, and flue gases are released. Carbon capture during post-combustion processes is considered a viable option to help reduce CO2 emissions.
Owing to the extensive study on CO2 capture in the post-combustion, different methods have been developed, including absorption/ scrubbing, adsorption, membrane separation, cryogenic distillation, and chemical looping combustion. The main disadvantage of absorption and cryogenic distillation technologies, which are the most commonly used methods for CO2 capture, is their high energy requirements for the separation of the gas. Alternatively, new strategies based on adsorption are emerging as the most promising technologies.
Adsorption using porous materials
Porous materials are critical in the development of alternative technologies for CO2 capture. Solid porous materials have been used at the industrial scale for decades. Among them, the most used are carbonaceous materials and zeolites, which are preferred due to their low cost, availability, and stability. However, selectivity is crucial to achieving efficient and cost-effective processes in gas separation applications.
In the quest for novel materials to fulfill this need, Metal-Organic Frameworks (MOFs) were discovered as highly porous solids. MOFs possess a regular, ordered structure. During the synthesis of MOFs, properties such as porosity, pore size and shape, and surface reactivity can be tuned easily, which is not readily possible for many other materials. MOFs' ability for easy tuning is an appealing feature that allows increasing selectivity towards CO2 over other gases.
CO2 capture with MOFs
Typical adsorption-desorption processes for capture and release of CO2 in porous materials are driven by thermal and pressure gradients (also known as temperature-swing adsorption (TSA) and pressure-swing adsorption (PSA)). In MOFs, the adsorption-desorption process is not limited to these driven forces. These hybrid materials open the possibilities to operate with novel stimuli-response methods such as:
- Photo-responsive regeneration
- Magnetic field responsive regeneration
- Electric field responsive regeneration
The responsiveness of light and magnetic sensitive MOFs is impressive and can reduce energy consumption in the capture-release process. Light triggered gas release in MOF systems achieves up to 90% CO2 desorption under ambient conditions. The combination of magnetic and light-triggered gas release has been tested for improved efficiencies. Compared to conventional methods such as absorption with liquid amines, MOF-based systems with the Magnetic-Light Induction Swing Adsorption method can reduce energy consumption by up to 80%. More details on the application of MOFs for CO2 capture are available in our success stories.
Integrated CO2 utilization
Once the CO2 is captured, it can be used for applications such as CO2 enrichment of greenhouse facilities. CO2 concentration is critical to achieving the maximum growth capacity of a crop. MOF-based materials can be coupled to this application to take advantage of low energy consumption for remote locations. Another possible application for the captured CO2 is the beverage industry, where carbon dioxide is used for carbonation.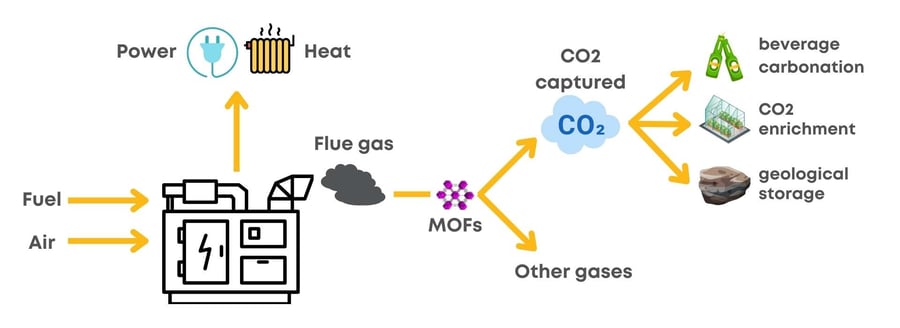 CO2 utilization is not limited to the applications mentioned above, where it can be used directly. CO2 transformation through chemical and catalytic processes can generate different value-added products such as methanol, urea, and polymers, among others.
CO2 utilization is not limited to the applications mentioned above, where it can be used directly. CO2 transformation through chemical and catalytic processes can generate different value-added products such as methanol, urea, and polymers, among others.
In light of Earth Day, we intend to draw a picture of the importance of CO2 capture, from the available capture technologies to the applications that can explode the carbon dioxide stored. In the big picture, MOFs are versatile materials that can be used during the overall process.




.jpg)
